Cla Content in Grass Fed Beef
- Review
- Open Access
- Published:
A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef
Nutrition Journal volume 9, Article number:10 (2010) Cite this article
Abstract
Growing consumer interest in grass-fed beef products has raised a number of questions with regard to the perceived differences in nutritional quality between grass-fed and grain-fed cattle. Research spanning three decades suggests that grass-based diets can significantly improve the fatty acid (FA) composition and antioxidant content of beefiness, albeit with variable impacts on overall palatability. Grass-based diets have been shown to raise total conjugated linoleic acid (CLA) (C18:2) isomers, trans vaccenic acid (TVA) (C18:1 t11), a precursor to CLA, and omega-3 (n-iii) FAs on a g/g fat footing. While the overall concentration of total SFAs is not different betwixt feeding regimens, grass-finished beef tends toward a higher proportion of cholesterol neutral stearic FA (C18:0), and less cholesterol-elevating SFAs such as myristic (C14:0) and palmitic (C16:0) FAs. Several studies suggest that grass-based diets elevate precursors for Vitamin A and Due east, equally well as cancer fighting antioxidants such as glutathione (GT) and superoxide dismutase (SOD) activity every bit compared to grain-fed contemporaries. Fat conscious consumers will also prefer the overall lower fat content of a grass-fed beef product. Even so, consumers should be enlightened that the differences in FA content volition also requite grass-fed beefiness a distinct grass season and unique cooking qualities that should be considered when making the transition from grain-fed beefiness. In add-on, the fat from grass-finished beef may have a yellowish appearance from the elevated carotenoid content (precursor to Vitamin A). Information technology is also noted that grain-fed beef consumers may attain similar intakes of both n-3 and CLA through the consumption of higher fat grain-fed portions.
Review Contents
- ane.
Introduction
- 2.
Fatty acid profile in grass-fed beef
- 3.
Impact of grass-finishing on omega-three fatty acids
- 4.
Impact of grass-finishing on conjugated linoleic acrid (CLA) and trans-vaccenic acid (TVA)
- 5.
Impact of grass-finishing on β-carotenes/carotenoids
- 6.
Touch of grass-finishing on α-tocopherol
- 7.
Bear upon of grass-finishing on GT & SOD activity
- viii.
Impact of grass-finishing on season and palatability
- ix.
Decision
- ten.
References
Introduction
There is considerable support among the nutritional communities for the nutrition-heart (lipid) hypothesis, the idea that an imbalance of dietary cholesterol and fats are the main cause of atherosclerosis and cardiovascular illness (CVD) [1]. Health professionals globe-wide recommend a reduction in the overall consumption of SFAs, trans-fatty acids (TAs) and cholesterol, while emphasizing the need to increase intake of n-3 polyunsaturated fats [1, ii]. Such wide sweeping nutritional recommendations with regard to fat consumption are largely due to epidemiologic studies showing strong positive correlations between intake of SFA and the incidence of CVD, a condition believed to event from the concomitant rising in serum low-density-lipoprotein (LDL) cholesterol as SFA intake increases [three, 4]. For example, it is generally accepted that for every i% increase in energy from SFA, LDL cholesterol levels reportedly increase by 1.three to 1.7 mg/dL (0.034 to 0.044 mmol/L) [5–7].
Wide promotion of this correlative data spurred an anti-SFA campaign that reduced consumption of dietary fats, including most animal proteins such as meat, dairy products and eggs over the last 3 decades [viii], indicted on their relatively loftier SFA and cholesterol content. However, more recent lipid research would suggest that not all SFAs have the aforementioned affect on serum cholesterol. For case, lauric acrid (C12:0) and myristic acid (C14:0), have a greater total cholesterol raising effect than palmitic acid (C16:0), whereas stearic acid (C18:0) has a neutral effect on the concentration of full serum cholesterol, including no apparent bear upon on either LDL or HDL. Lauric acid increases total serum cholesterol, although it too decreases the ratio of full cholesterol:HDL considering of a preferential increase in HDL cholesterol [5, 7, 9]. Thus, the individual fatty acrid profiles tend to be more instructive than broad lipid classifications with respect to subsequent impacts on serum cholesterol, and should therefore be considered when making dietary recommendations for the prevention of CVD.
Clearly the lipid hypothesis has had broad sweeping impacts; not only on the way we eat, simply also on the manner food is produced on-farm. Indeed, changes in animal convenance and genetics take resulted in an overall leaner beef product[10]. Preliminary examination of diets containing today'southward leaner beef has shown a reduction in serum cholesterol, provided that beef consumption is express to a three ounce portion devoid of all external fat [11]. O'Dea's work was the offset of several studies to show today'south leaner beefiness products can reduce plasma LDL concentrations in both normal and hyper-cholesterolemic subjects, theoretically reducing hazard of CVD [12–xv].
Beyond changes in genetics, some producers accept likewise altered their feeding practices whereby reducing or eliminating grain from the ruminant nutrition, producing a product referred to every bit "grass-fed" or "grass-finished". Historically, about of the beefiness produced until the 1940's was from cattle finished on grass. During the 1950'southward, considerable research was done to improve the efficiency of beef production, giving birth to the feedlot industry where high free energy grains are fed to cattle equally means to subtract days on feed and improve marbling (intramuscular fat: International monetary fund). In addition, U.S. consumers take grown accustomed to the taste of grain-fed beef, generally preferring the flavor and overall palatability afforded by the higher free energy grain ration[16]. However, changes in consumer demand, coupled with new research on the issue of feed on nutrient content, accept a number of producers returning to the pastoral arroyo to beef production despite the inherent inefficiencies.
Research spanning three decades suggests that grass-only diets can significantly modify the fat acrid composition and improve the overall antioxidant content of beef. It is the intent of this review, to synthesize and summarize the information currently available to substantiate an enhanced nutrient claim for grass-fed beef products too every bit to hash out the effects these specific nutrients have on human being wellness.
Review of fatty acrid profiles in grass-fed beef
Red meat, regardless of feeding regimen, is nutrient dense and regarded equally an important source of essential amino acids, vitamins A, B6, B12, D, E, and minerals, including fe, zinc and selenium [17, 18]. Along with these important nutrients, meat consumers also ingest a number of fats which are an of import source of energy and facilitate the absorption of fat-soluble vitamins including A, D, E and One thousand. According to the ADA, fauna fats contribute approximately lx% of the SFA in the American diet, most of which are palmitic acid (C16:0) and stearic acid (C18:0). Stearic acid has been shown to take no net impact on serum cholesterol concentrations in humans[17, 19]. In addition, thirty% of the FA content in conventionally produced beef is equanimous of oleic acrid (C18:1) [20], a monounsaturated FA (MUFA) that elicits a cholesterol-lowering effect among other healthful attributes including a reduced chance of stroke and a significant decrease in both systolic and diastolic blood force per unit area in susceptible populations [21].
Exist that as information technology may, changes in finishing diets of conventional cattle tin modify the lipid profile in such a way as to improve upon this nutritional parcel. Although in that location are genetic, age related and gender differences among the various meat producing species with respect to lipid profiles and ratios, the effect of animal diet is quite significant [22]. Regardless of the genetic makeup, gender, age, species or geographic location, direct contrasts betwixt grass and grain rations consistently demonstrate significant differences in the overall fat acid profile and antioxidant content constitute in the lipid depots and torso tissues [22–24].
Table one summarizes the saturated fatty acid analysis for a number of studies whose objectives were to contrast the lipid profiles of cattle fed either a grain or grass diets [25–31]. This table is express to those studies utilizing the longissimus dorsi (loin center), thereby standardizing the contrasts to similar cuts inside the carcass and limits the comparisons to cattle between twenty and 30 months of age. Unfortunately, not all studies report information in like units of measure out (i.due east., g/one thousand of fatty acrid), and then directly comparisons between studies are non possible.
Table i reports that grass finished cattle are typically lower in total fat as compared to grain-fed contemporaries. Interestingly, in that location is no consistent difference in total SFA content betwixt these ii feeding regimens. Those SFA's considered to be more detrimental to serum cholesterol levels, i.eastward., myristic (C14:0) and palmitic (C16:0), were higher in grain-fed beef as compared to grass-fed contemporaries in 60% of the studies reviewed. Grass finished meat contains elevated concentrations of stearic acid (C18:0), the merely saturated fat acid with a net neutral impact on serum cholesterol. Thus, grass finished beefiness tends to produce a more favorable SFA composition although lilliputian is known of how grass-finished beef would ultimately bear on serum cholesterol levels in hyper-cholesterolemic patients as compared to a grain-fed beefiness.
Like SFA intake, dietary cholesterol consumption has likewise go an of import issue to consumers. Interestingly, beefiness's cholesterol content is similar to other meats (beefiness 73; pork 79; lamb 85; chicken 76; and turkey 83 mg/100 chiliad) [32], and can therefore be used interchangeably with white meats to reduce serum cholesterol levels in hyper-cholesterolemic individuals[11, 33]. Studies have shown that brood, nutrition and sex exercise not touch the cholesterol concentration of bovine skeletal muscle, rather cholesterol content is highly correlated to IMF concentrations[34]. Every bit IMF levels rise, so goes cholesterol concentrations per gram of tissue [35]. Because pasture raised beefiness is lower in overall fat [24–27, 30], peculiarly with respect to marbling or IMF [26, 36], it would seem to follow that grass-finished beef would be lower in overall cholesterol content although the data is very limited. Garcia et al (2008) report twoscore.3 and 45.eight grams of cholesterol/100 grams of tissue in pastured and grain-fed steers, respectively (P < 0.001) [24].
Interestingly, grain-fed beefiness consistently produces higher concentrations of MUFAs as compared to grass-fed beef, which include FAs such as oleic acid (C18:i cis-9), the primary MUFA in beef. A number of epidemiological studies comparing disease rates in unlike countries have suggested an inverse association between MUFA intake and bloodshed rates to CVD [3, 21]. Even so, grass-fed beef provides a college concentration of TVA (C18:1 txi), an important MUFA for de novo synthesis of conjugated linoleic acid (CLA: C18:2 c-9, t-eleven), a potent anti-carcinogen that is synthesized within the torso tissues [37]. Specific data relative to the health benefits of CLA and its biochemistry will exist detailed later.
The important polyunsaturated fatty acids (PUFAs) in conventional beefiness are linoleic acid (C18:2), blastoff-linolenic acid (C18:3), described equally the essential FAs, and the long-chain fatty acids including arachidonic acid (C20:4), eicosapentaenoic acrid (C20:v), docosanpetaenoic acid (C22:v) and docosahexaenoic acid (C22:half-dozen) [38]. The significance of diet on fat acid composition is conspicuously demonstrated when profiles are examined by omega 6 (n-6) and omega 3 (n-3) families. Tabular array 2 shows no significant alter to the overall concentration of north-6 FAs between feeding regimens, although grass-fed beef consistently shows a college concentrations of north-iii FAs every bit compared to grain-fed contemporaries, creating a more than favorable northward-6:n-3 ratio. There are a number of studies that report positive effects of improved n-3 intake on CVD and other health related issues discussed in more item in the next section.
Review of Omega-3: Omega-half-dozen fatty acrid content in grass-fed beef
There are ii essential fat acids (EFAs) in human nutrition: α-linolenic acrid (αLA), an omega-3 fatty acrid; and linoleic acrid (LA), an omega-6 fat acid. The human body cannot synthesize essential fatty acids, even so they are disquisitional to human being health; for this reason, EFAs must be obtained from food. Both αLA and LA are polyunsaturated and serve as precursors of other important compounds. For instance, αLA is the precursor for the omega-3 pathway. Likewise, LA is the parent fat acid in the omega-vi pathway. Omega-iii (north-3) and omega-half dozen (n-6) fat acids are two separate distinct families, yet they are synthesized by some of the same enzymes; specifically, delta-v-desaturase and delta-6-desaturase. Excess of ane family of FAs can interfere with the metabolism of the other, reducing its incorporation into tissue lipids and altering their overall biological effects [39]. Effigy 1 depicts a schematic of northward-6 and due north-3 metabolism and elongation within the torso [40].
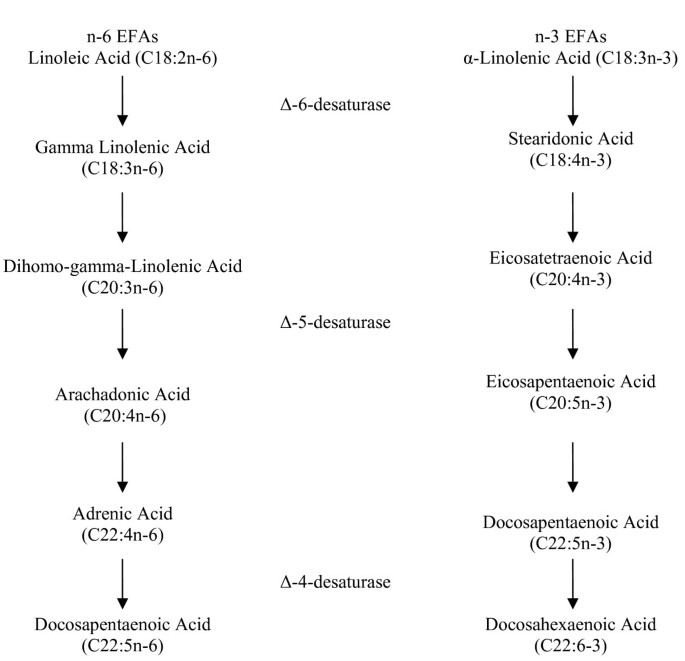
Linoleic (C18:2n-6) and α-Linolenic (C18:3n-iii) Acid metabolism and elongation. (Adjusted from Simopoulos et al., 1991)
A healthy diet should consist of roughly one to four times more omega-six fat acids than omega-3 fatty acids. The typical American diet tends to comprise 11 to 30 times more omega -half dozen fatty acids than omega -3, a phenomenon that has been hypothesized as a significant factor in the rising rate of inflammatory disorders in the United States[40]. Table 2 shows significant differences in n-6:due north-iii ratios between grass-fed and grain-fed beef, with and overall average of 1.53 and 7.65 for grass-fed and grain-fed, respectively, for all studies reported in this review.
The major types of omega-3 fatty acids used by the body include: α-linolenic acrid (C18:3n-3, αLA), eicosapentaenoic acid (C20:5n-3, EPA), docosapentaenoic acrid (C22:5n-iii, DPA), and docosahexaenoic acid (C22:6n-3, DHA). In one case eaten, the torso converts αLA to EPA, DPA and DHA, albeit at low efficiency. Studies by and large agree that whole torso conversion of αLA to DHA is beneath 5% in humans, the bulk of these long-chain FAs are consumed in the nutrition [41].
The omega-3 fatty acids were get-go discovered in the early on 1970's when Danish physicians observed that Greenland Eskimos had an exceptionally low incidence of heart illness and arthritis despite the fact that they consumed a diet high in fat. These early studies established fish as a rich source of n-three fatty acids. More than recent research has established that EPA and DHA play a crucial role in the prevention of atherosclerosis, centre assail, depression and cancer [40, 42]. In improver, omega-three consumption reduced the inflammation acquired by rheumatoid arthritis [43, 44].
The man brain has a high requirement for DHA; low DHA levels take been linked to depression brain serotonin levels, which are continued to an increased trend for depression and suicide. Several studies take established a correlation betwixt low levels of omega -three fatty acids and depression. High consumption of omega-3 FAs is typically associated with a lower incidence of depression, a decreased prevalence of historic period-related retention loss and a lower risk of developing Alzheimer's affliction [45–51].
The National Institutes of Wellness has published recommended daily intakes of FAs; specific recommendations include 650 mg of EPA and DHA, two.22 g/day of αLA and iv.44 thousand/twenty-four hours of LA. Still, the Institute of Medicine has recommended DRI (dietary reference intake) for LA (omega-six) at 12 to 17 yard and αLA (omega-3) at 1.1 to i.vi chiliad for adult women and men, respectively. Although seafood is the major dietary source of northward-3 fatty acids, a recent fat acid intake survey indicated that cherry-red meat also serves as a meaning source of n-iii fatty acids for some populations [52].
Sinclair and co-workers were the showtime to show that beef consumption increased serum concentrations of a number of n-3 fatty acids including, EPA, DPA and DHA in humans [twoscore]. Too, there are a number of studies that have been conducted with livestock which report similar findings, i.due east., animals that eat rations high in precursor lipids produce a meat product higher in the essential fatty acids [53, 54]. For example, cattle fed primarily grass significantly increased the omega-3 content of the meat and besides produced a more favorable omega-six to omega-three ratio than grain-fed beef [46, 55–57].
Table 2 shows the effect of ration on polyunsaturated fatty acid composition from a number of contempo studies that contrast grass-based rations to conventional grain feeding regimens [24–28, xxx, 31]. Grass-based diets resulted in significantly higher levels of omega-3 inside the lipid fraction of the meat, while omega-6 levels were left unchanged. In fact, as the concentration of grain is increased in the grass-based diet, the concentration of due north-3 FAs decreases in a linear mode. Grass-finished beef consistently produces a higher concentration of due north-3 FAs (without effecting northward-6 FA content), resulting in a more favorable n-vi:n-three ratio.
The amount of total lipid (fat) constitute in a serving of meat is highly dependent upon the feeding regimen every bit demonstrated in Tables i and 2. Fat will also vary past cut, as not all locations of the carcass volition deposit fat to the same caste. Genetics as well play a role in lipid metabolism creating significant brood effects. Fifty-fifty and then, the effect of feeding regimen is a very powerful determinant of fatty acid composition.
Review of conjugated linoleic acrid (CLA) and transvaccenic acid (TVA) in grass-fed beefiness
Conjugated linoleic acids make up a grouping of polyunsaturated FAs found in meat and milk from ruminant animals and exist equally a general mixture of conjugated isomers of LA. Of the many isomers identified, the cis-nine, trans-eleven CLA isomer (also referred to equally rumenic acid or RA) accounts for upward to 80-90% of the total CLA in ruminant products [58]. Naturally occurring CLAs originate from two sources: bacterial isomerization and/or biohydrogenation of polyunsaturated fat acids (PUFA) in the rumen and the desaturation of trans-fat acids in the adipose tissue and mammary gland [59, 60].
Microbial biohydrogenation of LA and αLA by an anaerobic rumen bacterium Butyrivibrio fibrisolvens is highly dependent on rumen pH [61]. Grain consumption decreases rumen pH, reducing B. fibrisolven action, conversely grass-based diets provide for a more favorable rumen environment for subsequent bacterial synthesis [62]. Rumen pH may help to explicate the credible differences in CLA content between grain and grass-finished meat products (meet Table 2). De novo synthesis of CLA from 11t-C18:1 TVA has been documented in rodents, dairy cows and humans. Studies suggest a linear increment in CLA synthesis equally the TVA content of the diet increased in homo subjects [63]. The rate of conversion of TVA to CLA has been estimated to range from 5 to 12% in rodents to 19 to 30% in humans[64]. True dietary intake of CLA should therefore consider native 9c11t-C18:2 (actual CLA) as well as the xit-C18:1 (potential CLA) content of foods [65, 66]. Figure two portrays de novo synthesis pathways of CLA from TVA [37].
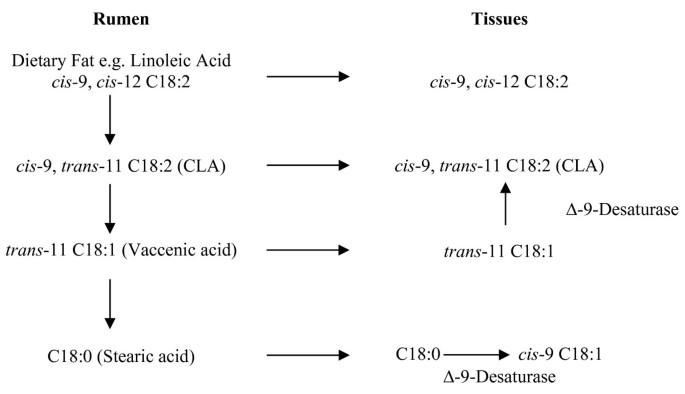
De novo synthesis of CLA from 11t-C18:1 vaccenic acid. (Adapted from Bauman et al., 1999)
Natural augmentation of CLA cixtxi and TVA inside the lipid fraction of beef products tin exist accomplished through diets rich in grass and lush green forages. While precursors can be found in both grains and lush green forages, grass-fed ruminant species have been shown to produce 2 to iii times more CLA than ruminants fed in solitude on high grain diets, largely due to a more favorable rumen pH [34, 56, 57, 67] (see Table 2).
The touch on of feeding practices becomes fifty-fifty more than axiomatic in lite of recent reports from Canada which suggests a shift in the predominate trans C18:i isomer in grain-fed beef. Dugan et al (2007) reported that the major trans isomer in beefiness produced from a 73% barley grain diet is 10t-18:1 (2.13% of total lipid) rather than 11t-eighteen:1 (TVA) (0.77% of full lipid), a finding that is non peculiarly favorable because the data that would support a negative touch of 10t-18:1 on LDL cholesterol and CVD [68, 69].
Over the past two decades numerous studies have shown significant health benefits attributable to the actions of CLA, as demonstrated by experimental brute models, including deportment to reduce carcinogenesis, atherosclerosis, and onset of diabetes [70–72]. Conjugated linoleic acid has too been reported to modulate body limerick by reducing the aggregating of adipose tissue in a multifariousness of species including mice, rats, pigs, and now humans [73–76]. These changes in body limerick occur at ultra high doses of CLA, dosages that tin only exist attained through synthetic supplementation that may also produce ill side-effects, such as gastrointestinal upset, adverse changes to glucose/insulin metabolism and compromised liver part [77–81]. A number of fantabulous reviews on CLA and human being health can be institute in the literature [61, 82–84].
Optimal dietary intake remains to be established for CLA. It has been hypothesized that 95 mg CLA/day is enough to show positive effects in the reduction of breast cancer in women utilizing epidemiological data linking increased milk consumption with reduced breast cancer[85]. Ha et al. (1989) published a much more bourgeois estimate stating that three 1000/day CLA is required to promote human health benefits[86]. Ritzenthaler et al. (2001) estimated CLA intakes of 620 mg/day for men and 441 mg/day for women are necessary for cancer prevention[87]. Plain, all these values represent rough estimates and are mainly based on extrapolated creature data. What is articulate is that we as a population exercise not consume plenty CLA in our diets to have a pregnant touch on cancer prevention or suppression. Reports indicate that Americans consume between 150 to 200 mg/24-hour interval, Germans consumer slightly more between 300 to 400 mg/day[87], and the Australians seem to exist closer to the optimum concentration at 500 to thou mg/mean solar day according to Parodi (1994) [88].
Review of pro-Vitamin A/β-carotene in grass-fed meat
Carotenoids are a family of compounds that are synthesized by college plants as natural plant pigments. Xanthophylls, carotene and lycopene are responsible for yellowish, orangish and reddish coloring, respectively. Ruminants on high fodder rations pass a portion of the ingested carotenoids into the milk and body fatty in a manner that has still to be fully elucidated. Cattle produced under extensive grass-based production systems generally accept carcass fat which is more yellow than their concentrate-fed counterparts caused past carotenoids from the lush light-green forages. Although yellow carcass fatty is negatively regarded in many countries around the globe, it is also associated with a healthier fatty acid profile and a higher antioxidant content [89].
Institute species, harvest methods, and season, all take significant impacts on the carotenoid content of forage. In the process of making silage, haylage or hay, as much as 80% of the carotenoid content is destroyed [xc]. Further, meaning seasonal shifts occur in carotenoid content owing to the seasonal nature of plant growth.
Carotenes (mainly β-carotene) are precursors of retinol (Vitamin A), a critical fat-soluble vitamin that is important for normal vision, bone growth, reproduction, cell division, and cell differentiation [91]. Specifically, information technology is responsible for maintaining the surface lining of the optics and also the lining of the respiratory, urinary, and intestinal tracts. The overall integrity of peel and mucous membranes is maintained by vitamin A, creating a barrier to bacterial and viral infection [15, 92]. In add-on, vitamin A is involved in the regulation of immune function by supporting the production and function of white claret cells [12, thirteen].
The current recommended intake of vitamin A is 3,000 to 5,000 IU for men and 2,300 to four,000 IU for women [93], respectively, which is equivalent to 900 to 1500 μg (micrograms) (Note: DRI as reported by the Institute of Medicine for non-meaning/non-lactating developed females is 700 μg/day and males is 900 μg/day or 2,300 - 3,000 I U (bold conversion of 3.33 IU/μg). While there is no RDA (Required Daily Allowance) for β-carotene or other pro-vitamin A carotenoids, the Plant of Medicine suggests consuming three mg of β-carotene daily to maintain plasma β-carotene in the range associated with normal function and a lowered risk of chronic diseases (NIH: Function of Dietary Supplements).
The furnishings of grass feeding on beta-carotene content of beef was described by Descalzo et al. (2005) who found pasture-fed steers incorporated significantly higher amounts of beta-carotene into muscle tissues as compared to grain-fed animals [94]. Concentrations were 0.45 μg/g and 0.06 μg/g for beef from pasture and grain-fed cattle respectively, demonstrating a 7 fold increase in β-carotene levels for grass-fed beefiness over the grain-fed contemporaries. Similar data has been reported previously, presumably due to the high β-carotene content of fresh grasses as compared to cereal grains[38, 55, 95–97]. (run across Table three)
Review of Vitamin E/α-tocopherol in grass-fed beef
Vitamin E is besides a fat-soluble vitamin that exists in eight different isoforms with powerful antioxidant activity, the near active being α-tocopherol [98]. Numerous studies accept shown that cattle finished on pasture produce higher levels of α-tocopherol in the final meat product than cattle fed high concentrate diets[23, 28, 94, 97, 99–101] (encounter Table iv).
Antioxidants such as vitamin E protect cells confronting the effects of complimentary radicals. Free radicals are potentially dissentious past-products of metabolism that may contribute to the development of chronic diseases such as cancer and cardiovascular disease.
Preliminary research shows vitamin Eastward supplementation may help forbid or filibuster coronary heart disease [102–105]. Vitamin E may also block the formation of nitrosamines, which are carcinogens formed in the stomach from nitrates consumed in the diet. Information technology may too protect against the development of cancers by enhancing immune function [106]. In addition to the cancer fighting furnishings, in that location are some observational studies that found lens clarity (a diagnostic tool for cataracts) was improve in patients who regularly used vitamin E [107, 108]. The electric current recommended intake of vitamin Due east is 22 IU (natural source) or 33 IU (synthetic source) for men and women [93, 109], respectively, which is equivalent to 15 milligrams past weight.
The concentration of natural α-tocopherol (vitamin E) found in grain-fed beef ranged between 0.75 to 2.92 μg/k of muscle whereas pasture-fed beefiness ranges from 2.1 to seven.73 μg/g of tissue depending on the blazon of fodder made bachelor to the animals (Tabular array four). Grass finishing increases α-tocopherol levels three-fold over grain-fed beefiness and places grass-fed beef well within range of the musculus α-tocopherol levels needed to extend the shelf-life of retail beef (iii to iv μg α-tocopherol/gram tissue) [110]. Vitamin E (α-tocopherol) acts post-mortem to filibuster oxidative deterioration of the meat; a process by which myoglobin is converted into brown metmyoglobin, producing a darkened, brown appearance to the meat. In a report where grass-fed and grain-fed beefiness were directly compared, the bright crimson color associated with oxymyoglobin was retained longer in the retail display in the grass-fed group, even idea the grass-fed meat contains a higher concentration of more oxidizable n-3 PUFA. The authors concluded that the antioxidants in grass probably caused higher tissue levels of vitamin E in grazed animals with benefits of lower lipid oxidation and amend colour memory despite the greater potential for lipid oxidation[111].
Review of antioxidant enzyme content in grass-fed beefiness
Glutathione (GT), is a relatively new protein identified in foods. It is a tripeptide equanimous of cysteine, glutamic acid and glycine and functions as an antioxidant primarily as a component of the enzyme arrangement containing GT oxidase and reductase. Within the cell, GT has the capability of quenching gratuitous radicals (like hydrogen peroxide), thus protecting the cell from oxidized lipids or proteins and forestall harm to Deoxyribonucleic acid. GT and its associated enzymes are establish in virtually all plant and beast tissue and is readily absorbed in the small intestine[112].
Although our knowledge of GT content in foods is still somewhat limited, dairy products, eggs, apples, beans, and rice comprise very little GT (< three.3 mg/100 g). In dissimilarity, fresh vegetables (e.g., asparagus 28.iii mg/100 g) and freshly cooked meats, such as ham and beefiness (23.iii mg/100 g and 17.5 mg/100 k, respectively), are high in GT [113].
Because GT compounds are elevated in lush greenish forages, grass-fed beef is particularly high in GT equally compared to grain-fed contemporaries. Descalzo et al. (2007) reported a significant increase in GT molar concentrations in grass-fed beef [114]. In improver, grass-fed samples were also higher in superoxide dismutase (SOD) and catalase (CAT) activity than beef from grain-fed animals[115]. Superoxide dismutase and catalase are coupled enzymes that work together every bit powerful antioxidants, SOD scavenges superoxide anions by forming hydrogen peroxide and Cat and then decomposes the hydrogen peroxide to H2O and O2. Grass only diets improve the oxidative enzyme concentration in beefiness, protecting the muscle lipids confronting oxidation also as providing the beef consumer with an additional source of antioxidant compounds.
Issues related to flavor and palatability of grass-fed beef
Maintaining the more favorable lipid profile in grass-fed beefiness requires a high percent of lush fresh forage or grass in the ration. The higher the concentration of fresh green forages, the higher the αLA forerunner that will be available for CLA and n-3 synthesis [53, 54]. Fresh pasture forages have ten to 12 times more C18:3 than cereal grains [116]. Dried or cured forages, such equally hay, will take a slightly lower corporeality of forerunner for CLA and n-3 synthesis. Shifting diets to cereal grains will crusade a significant change in the FA contour and antioxidant content inside 30 days of transition [57].
Because grass-finishing alters the biochemistry of the beef, aroma and season will also be affected. These attributes are straight linked to the chemical makeup of the final product. In a study comparing the flavor compounds betwixt cooked grass-fed and grain-fed beef, the grass-fed beef independent higher concentrations of diterpenoids, derivatives of chlorophyll call phyt-1-ene and phyt-2-ene, that changed both the flavor and odour of the cooked product [117]. Others have identified a "green" odor from cooked grass-fed meat associated with hexanals derived from oleic and αLA FAs. In contrast to the "light-green" aroma, grain-fed beef was described equally possessing a "soapy" aroma, presumably from the octanals formed from LA that is constitute in loftier concentration in grains [118]. Grass-fed beef consumers can expect a dissimilar flavor and aroma to their steaks as they cook on the grill. Likewise, because of the lower lipid content and high concentration of PUFAs, cooking fourth dimension will be reduced. For an exhaustive wait at the effect of meat compounds on flavor, see Calkins and Hodgen (2007) [119].
With respect to palatability, grass-fed beef has historically been less well accepted in markets where grain-fed products predominant. For example, in a report where British lambs fed grass and Spanish lambs fed milk and concentrates were assessed past British and Spanish sense of taste panels, both establish the British lamb to have a higher odor and season intensity. Withal, the British panel preferred the flavor and overall eating quality of the grass-fed lamb, the Spanish panel much preferred the Spanish fed lamb [120]. Besides, the U.South. is well known for producing corn-fed beef, taste panels and consumers who are more familiar with the taste of corn-fed beef seem to adopt information technology also [16]. An individual usually comes to prefer the foods they grew up eating, making consumer sensory panels more of an fine art than science [36]. Trained taste panels, i.east., persons specifically trained to evaluate sensory characteristics in beef, plant grass-fed beef less palatable than grain-fed beef in flavour and tenderness [119, 121].
Conclusion
Research spanning three decades supports the statement that grass-fed beef (on a g/g fat basis), has a more than desirable SFA lipid profile (more C18:0 cholesterol neutral SFA and less C14:0 & C16:0 cholesterol elevating SFAs) every bit compared to grain-fed beef. Grass-finished beef is also college in total CLA (C18:2) isomers, TVA (C18:1 t11) and n-3 FAs on a g/g fat basis. This results in a better n-6:northward-3 ratio that is preferred by the nutritional community. Grass-fed beef is likewise higher in precursors for Vitamin A and Due east and cancer fighting antioxidants such as GT and SOD activity as compared to grain-fed contemporaries.
Grass-fed beef tends to be lower in overall fatty content, an important consideration for those consumers interested in decreasing overall fatty consumption. Because of these differences in FA content, grass-fed beef also possesses a singled-out grass flavour and unique cooking qualities that should exist considered when making the transition from grain-fed beefiness. To maximize the favorable lipid contour and to guarantee the elevated antioxidant content, animals should be finished on 100% grass or pasture-based diets.
Grain-fed beef consumers may attain similar intakes of both northward-3 and CLA through consumption of higher fat portions with higher overall palatability scores. A number of clinical studies accept shown that today's lean beef, regardless of feeding strategy, can exist used interchangeably with fish or skinless chicken to reduce serum cholesterol levels in hypercholesterolemic patients.
Abbreviations
- c:
-
cis
- t:
-
trans
- FA:
-
fat acid
- SFA:
-
saturated fatty acid
- PUFA:
-
polyunsaturated fatty acid
- MUFA:
-
monounsaturated fatty acid
- CLA:
-
conjugated linoleic acid
- TVA:
-
trans-vaccenic acid
- EPA:
-
eicosapentaenoic acid
- DPA:
-
docosapentaenoic acid
- DHA:
-
docosahexaenoic acid
- GT:
-
glutathione
- SOD:
-
superoxide dismutase
- CAT:
-
catalase.
References
-
Griel AE, Kris-Etherton PM: Beyond saturated fatty: The importance of the dietary fatty acid contour on cardiovascular affliction. Nutrition Reviews. 2006, 64 (5): 257-62. 10.1111/j.1753-4887.2006.tb00208.x.
-
Kris-Etherton PM, Innis S: Dietary Fatty Acids -- Position of the American Dietetic Association and Dietitians of Canada. American Dietetic Association Position Written report. Journal of the American Dietetic Association. 2007, 107 (nine): 1599-1611. Ref Type: Report
-
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekins CH, Willett WC: Dietary fat intake and the gamble of coronary center disease in women. New England Journal of Medicine. 1997, 337: 1491-9. 10.1056/NEJM199711203372102.
-
Posner BM, Cobb JL, Belanger AJ, Cupples LA, D'Agostino RB, Stokes J: Dietary lipid predictors of coronary center disease in men. The Framingham Report. Athenaeum of Internal Medicine. 1991, 151: 1181-7. x.1001/archinte.151.6.1181.
-
Mensink RP, Katan MB: Effect of dietary fat acids on serum lipids and lipoproteins. Arteriosclerosis Thrombosis Vascular Biology. 1992, 12: 911-9.
-
Keys A: Coronary heart affliction in 7 countries. Apportionment. 1970, 41 (1): 211-
-
Mensink RP, Zock PL, Kester AD, Katan MB: Effects of dietary fat acids and carbohydrates on the ratio of serum total HDL cholesterol and on serum lipids and apolipoproteins: A meta-assay of threescore controlled trials. American Journal of Clinical Nutrition. 2003, 77: 1146-55.
-
Putnam J, Allshouse J, Scott-Kantor L: U.Southward. per capita nutrient supply trends: More than calories, refined carbohydrates, and fats. Food Review. 2002, 25 (3): 2-15.
-
Kris-Etherton PMYS: Individual fatty acid effects on plasma lipids and lipoproteins. Human being studies. American Journal of Clinical Nutrition. 1997, 65 (suppl.five): 1628S-44S.
-
Higgs JD: The changing nature of red meat: xx years improving nutritional quality. Trends in Food Scientific discipline and Technology. 2000, 11: 85-95. ten.1016/S0924-2244(00)00055-eight.
-
O'Dea K, Traianedes K, Chisholm K, Leyden H, Sinclair AJ: Cholesterol-lowering effect of a low-fat nutrition containing lean beefiness is reversed by the addition of beefiness fatty. American Journal of Clinical Diet. 1990, 52: 491-iv.
-
Beauchesne-Rondeau Eastward, Gascon A, Bergeron J, Jacques H: Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. American Periodical of Clinical Diet. 2003, 77 (3): 587-93.
-
Melanson One thousand, Gootman J, Myrdal A, Kline Chiliad, Rippe JM: Weight loss and total lipid profile changes in overweight women consuming beef or chicken as the primary protein source. Nutrition. 2003, 19: 409-14. 10.1016/S0899-9007(02)01080-8.
-
Denke MA: Part of beef and beefiness tallow, an enriched source of stearic acid, in a cholesterol-lowering diet. American Journal of Clinical Diet. 1994, 60: 1044S-9S.
-
Smith DR, Wood R, Tseng South, Smith SB: Increased beefiness consumption increases lipoprotein A-I simply not serum cholesterol of mildly hypercholesterolemic men with dissimilar levels of habitual beef intake. Experimental Biological Medicine. 2002, 227 (4): 266-75.
-
Woods JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser Grand: Effects of fatty acids on meat quality: review. Meat Science. 2003, 66: 21-32. 10.1016/S0309-1740(03)00022-six.
-
Williamson CS, Foster RK, Stanner SA, Buttriss JL: Blood-red meat in the diet. British Nutrition Foundation. Diet Bulletin. 2005, xxx: 323-335. 10.1111/j.1467-3010.2005.00525.ten. Ref Type: Written report
-
Biesalski HK: Meat every bit a component of a salubrious diet - are in that location any risks or benefits if meat is avoided?. Meat Science. 2005, 70 (3): 509-24. 10.1016/j.meatsci.2004.07.017.
-
Yu S, Derr J, Etherton TD, Kris-Etherton PM: Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monosaturated fatty acids are hypocholesterolemic. American Journal of Clinical Nutrition. 1995, 61: 1129-39.
-
Whetsell MS, Rayburn EB, Lozier JD: Human Health Effects of Fatty Acids in Beef. 2003, Fact Sheet: West Virgina Academy & U.S.D.A. Agronomics Research Service. Extension Service West Virginia University, Ref Type: Electronic Citation
-
Kris-Etherton PM: Monounsaturated fatty acids and hazard of cardiovascular affliction. Circulation. 1999, 100: 1253-
-
DeSmet Southward, Raes 1000, Demeyer D: Meat fatty acid composition as afflicted past fatness and genetic factors: a review. Animate being Research. 2004, 53: 81-98. 10.1051/animres:2004003.
-
De la Fuente J, Diaz MT, Alvarez I, Oliver MA, Font i Furnols One thousand, Sanudo C, Campo MM, Montossi F, Nute GR, Caneque V: Fatty acid and vitamin E limerick of intramuscular fat in cattle reared in unlike production systems. Meat Science. 2009, 82: 331-7. 10.1016/j.meatsci.2009.02.002.
-
Garcia PT, Pensel NA, Sancho AM, Latimori NJ, Kloster AM, Amigone MA, Casal JJ: Beefiness lipids in relation to animal breed and nutrition in Argentina. Meat Scientific discipline. 2008, 79: 500-eight. 10.1016/j.meatsci.2007.x.019.
-
Alfaia CPM, Alves SP, Martins SIV, Costa ASH, Fontes CMGA, Lemos JPC, Bessa RJB, Prates JAM: Effect of feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory power. Food Chemical science. 2009, 114: 939-46. 10.1016/j.foodchem.2008.10.041.
-
Leheska JM, Thompson LD, Howe JC, Hentges E, Boyce J, Brooks JC, Shriver B, Hoover L, Miller MF: Effects of conventional and grass-feeding systems on the nutrient composition of beef. Periodical Animal Science. 2008, 86: 3575-85. x.2527/jas.2007-0565.
-
Nuernberg K, Dannenberger D, Nuernberg Yard, Ender K, Voigt J, Scollan ND, Wood JD, Nute GR, Richardson RI: Upshot of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid limerick of longissimus muscle in different cattle breeds. Livestock Production Science. 2005, 94: 137-47. x.1016/j.livprodsci.2004.11.036.
-
Realini CE, Duckett SK, Brito GW, Rizza MD, De Mattos D: Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beefiness. Meat Science. 2004, 66: 567-77. 10.1016/S0309-1740(03)00160-8.
-
Warren HE, Enser One thousand, Richardson I, Wood JD, Scollan ND: Effect of breed and diet on total lipid and selected shelf-life parameters in beefiness muscle. Proceedings of British Society of animate being science. 2003, 23-
-
Ponnampalam EN, Mann NJ, Sinclair AJ: Result of feeding systems on omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beefiness cuts, potential bear upon on human health. Asia Pacific Periodical of Clinical Diet. 2006, fifteen (i): 21-nine.
-
Descalzo A, Insani EM, Biolatto A, Sancho AM, Garcia PT, Pensel NA: Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beefiness. Meat Science. 2005, 70: 35-44. 10.1016/j.meatsci.2004.11.018.
-
Wheeler TL, Davis GW, Stoecker BJ, Harmon CJ: Cholesterol concentrations of longissimus musculus, subcutaneous fat and serum of 2 beef cattle brood types. Journal of Animal Scientific discipline. 1987, 65: 1531-7.
-
Smith DR, Wood R, Tseng South, Smith SB: Increased beef consumption increases apolipoprotein A-1 just not serum cholesterol of mildly hypercholesterolemic men with dissimilar levels of habitual beef intake. Experimental Biological Medicine. 2002, 227 (iv): 266-75.
-
Dominion DC, Broughton KS, Shellito SM, Maiorano G: Comparison of muscle fat acrid profiles and cholesterol concentrations of bison, cattle, elk and craven. Journal Animal Science. 2002, 80: 1202-eleven.
-
Alfaia CPM, Castro MLF, Martins SIV, Portugal APV, Alves SPA, Fontes CMGA: Influence of slaughter season and muscle type on faty acid composition, conjugated linoleic acrid isomeric distribution and nutritional quality of intramuscular fat in Arouquesa-PDO veal. Meat Scientific discipline. 2007, 76: 787-95. 10.1016/j.meatsci.2007.02.023.
-
Sitz BM, Calkins CR, Feuz DM, Umberger WJ, Eskridge KM: Consumer sensory acceptance and value of domestic, Canadian, and Australian grass-fed beef steaks. Periodical of Animal Science. 2005, 83: 2863-8.
-
Bauman DE, Lock AL: Conjugated linoleic acid: biosynthesis and nutritional significance. Fox and McSweeney. Advanced Dairy Chemical science. 2006, Springer, New York, 93-136. Ref Type: Serial (Book, Monograph), 3, ii
-
Enser K, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington Thou: Fatty acid content and limerick of United kingdom beef and lamb muscle in relation to product system and implications for human nutrition. Meat Science. 1998, 49 (3): 329-41. 10.1016/S0309-1740(97)00144-7.
-
Ruxton CHS, Reed SC, Simpson JA, Millington KJ: The health benefits of omega-iii polyunsaturated fatty acids: a review of the evidence. The Journal of Human being Nutrition and Dietetics. 2004, 17: 449-59. 10.1111/j.1365-277X.2004.00552.x.
-
Simopoulos A: Omega-iii fatty acids in health and affliction and in growth and development. American Journal of Clinical Nutrition. 1991, 54: 438-63.
-
Thomas BJ: Efficiency of conversion of blastoff-linolenic acid to long chain n-iii fatty acids in human. Current Opinion in Clincal Nutrition and Metabolic Care. 2002, five (2): 127-32. ten.1097/00075197-200203000-00002.
-
Connor WE: Importance of n-3 fatty acids in health and disease. American Journal of Clinical Nutrition. 2000, 71: 171S-5S.
-
Kremer JM, Lawrence DA, Jubiz W, Galli C, Simopoulos AP: Different doses of fish -oil fat acid ingestion in agile rheumatoid arthritis: a prospective study of clinical and immunological parameters. Dietary Omega-3 and Omega-6 fatty acids: biological effects and nutritional essentiality. 1989, New York: Plenum Printing
-
DiGiacomo RA, Kremer JM, Shah DM: Fish-oil dietary supplementation in patients with Raynaud's Phenomenon: A double-blind, controlled, prospective study. The American Journal of Medicine. 1989, 86: 158-64. x.1016/0002-9343(89)90261-1.
-
Kalmijn S: Dietary fat intake and the risk of incident dementia in the Rotterdam Report. Annals of Neurology. 1997, 42 (5): 776-82. ten.1002/ana.410420514.
-
Yehuda S, Rabinovtz Southward, Carasso RL, Mostofsky DI: Essential fatty acids preparation (SR-3) improves Alzheimer'southward patient'south quality of life. International Journal of Neuroscience. 1996, 87 (3-4): 141-ix. 10.3109/00207459609070833.
-
Hibbeln JR: Fish oil consumption and major depression. The Lancet. 1998, 351: 1213-x.1016/S0140-6736(05)79168-6. (April 18 1998)
-
Hibbeln JR, Salem N: Dietary polyunsaturated fatty acids and depression: when cholesterol does non satisfy. American Periodical of Clinical Nutrition. 1995, 62: 1-9.
-
Stoll AL, et al: Omega iii fatty acids in bipolar disorder. Archives of General Psychiatry. 1999, 56: 407-12-415-sixteen
-
Calabrese JR, Rapport DJ, Shleton MD: Fish oils and bipolar disorder. Archives of Full general Psychiatry. 1999, 56: 413-four. 10.1001/archpsyc.56.5.413.
-
Laugharne JDE: Fat acids and schizophrenia. Lipids. 1996, 31: S163-S165. 10.1007/BF02637070.
-
Sinclair AJ, Johnson 50, O'Dea K, Holman RT: Diets rich in lean beef increase arachidonic acid and long-chain omega three polyunsaturated fatty acid levels in plasma phospholipids. Lipids. 1994, 29 (5): 337-43. ten.1007/BF02537187.
-
Raes K, DeSmet Due south, Demeyer D: Effect of dietary fat acids on incorporation of long chain polyunsaturated fat acids and conjugated linoleic acid in lamb, beef and pork meat: a review. Brute Feed Scientific discipline and Technology. 2004, 113: 199-221. 10.1016/j.anifeedsci.2003.09.001.
-
Marmer WN, Maxwell RJ, Williams JE: Effects of dietary regimen and tissue site on bovine fatty acrid profiles. Journal Brute Science. 1984, 59: 109-21.
-
Wood JD, Enser M: Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. British Journal of Diet. 1997, 78: S49-S60. 10.1079/BJN19970134.
-
French P, Stanton C, Lawless F, O'Riordan EG, Monahan FJ, Caffery PJ, Moloney AP: Fatty acid composition, including conjugated linoleic acid of intramuscular fat from steers offered grazed grass, grass silage or concentrate-based diets. Periodical Animal Scientific discipline. 2000, 78: 2849-55.
-
Duckett SK, Wagner DG, Yates LD, Dolezal HG, May SG: Furnishings of time on feed on beef nutrient composition. Journal Creature Science. 1993, 71: 2079-88.
-
Nuernberg K, Nuernberg K, Ender One thousand, Lorenz Southward, Winkler K, Rickert R, Steinhart H: Omega-3 fatty acids and conjugated linoleic acids of longissimus muscle in beefiness cattle. European Periodical of Lipid Science Technology. 2002, 104: 463-71. 10.1002/1438-9312(200208)104:viii<463::Help-EJLT463>3.0.CO;2-U.
-
Griinari JM, Corl BA, Lacy SH, Chouinard PY, Nurmela KV, Bauman DE: Conjugated linoleic acid is synthesized endogenoulsy in lactating dairy cows by delta-9 desaturase. Journal of Nutrition. 2000, 130: 2285-91.
-
Sehat North, Rickert RR, Mossoba MM, Dramer JKG, Yurawecz MP, Roach JAG, Adlof RO, Morehouse KM, Fritsche J, Eulitz KD, Steinhart H, Ku 1000: Improved separation of conjugated fatty acid methyl esters by silver ion-loftier-performance liquid chromatography. Lipids. 1999, 34: 407-13. 10.1007/s11745-999-0379-three.
-
Pariza MW, Park Y, Cook ME: Mechanisms of action of conjugated linoleic acid: bear witness and speculation. Proceedings for the Order of Experimental Biology and Medicine. 2000, 32: 853-8.
-
Bessa RJB, Santos-Silva J, Ribeiro JMR, Portugal AV: Reticulo-rumen biohydrogenation and the enrichment of ruminant edible products with linoleic acid conjugated isomers. Livestock Production Science. 2000, 63: 201-eleven. 10.1016/S0301-6226(99)00117-vii.
-
Turpeinen AM, Mutanen Thousand, Aro ASI, Basu SPD, Griinar JM: Bioconversion of vaccenic acid to conjugated linoleic acid in humans. American Periodical of Clinical Nutrition. 2002, 76: 504-10.
-
Turpeinen AM, Mautanen G, Aro A, Salminen I, Basu S, Palmquist DL: Bioconversion of vaccenic acid to conjugated linoleic acid in humans. American Journal of Clinical Diet. 2002, 76: 504-10.
-
Turpeinen AM, Mautanen M, Aro A, Salminen I, Basu Due south, Palmquist DL: Bioconversion of vaccenic acrid to conjugated linoleic acid in humans. American Journal of Clinical Nutrition. 2002, 76: 504-10.
-
Adlof RO, Duval Southward, Emken EA: Biosynthesis of conjugated linoleic acid in humans. Lipids. 2000, 35: 131-5. 10.1007/BF02664761.
-
Mandell IB, Gullett JG, Buchanan-Smith JG, Campbell CP: Effects of diet and slaughter endpoint on carcass composition and beef quality in Charolais cantankerous steers fed alfalfa silage and (or) high concentrate diets. Canadian Journal of Animal Science. 1997, 77: 403-xiv.
-
Dugan MER, Rollan DC, Aalhus JL, Aldai N, Kramer JKG: Subcutaneous fat composition of youthful and mature Canadian beefiness: emphasis on individual conjugated linoleic acid and trans-18:1 isomers. Canadian Journal of Animal Scientific discipline. 2008, 88: 591-9.
-
Hodgson JM, Wahlqvist ML, Boxall JA, Balazs ND: Platelet trans fat acids in relation to angiographically assessed coronary artery disease. Atherosclerosis. 1996, 120: 147-54. ten.1016/0021-9150(95)05696-3.
-
IP C, Scimeca JA, Thompson HJ: Conjugated linoleic acid. Cancer Supplement. 1994, 74 (iii): 1050-four.
-
Kritchevsky D, Tepper SA, Wright S, Tso P, Czarnecki SK: Influence of conjugated linoleic acrid (CLA) on establishment and progression of atherosclerosis in rabbits. Journal American Collection of Diet. 2000, xix (four): 472S-7S.
-
Steinhart H, Rickert R, Winkler K: Identification and analysis of conjugated linoleic acid isomers (CLA). European Journal of Medicine. 1996, xx (eight): 370-2.
-
Dugan MER, Aalhus JL, Jeremiah LE, Kramer JKG, Schaefer AL: The furnishings of feeding conjugated linoleic acrid on subsequent port quality. Canadian Journal of Animal Scientific discipline. 1999, 79: 45-51.
-
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW: Effect of conjugated linoleic acid on trunk composition in mice. Lipids. 1997, 32: 853-viii. 10.1007/s11745-997-0109-x.
-
Sisk M, Hausman D, Martin R, Azain Thou: Dietary conjugated linoleic acrid reduces adiposity in lean simply not obese Zucker rats. Journal of Diet. 2001, 131: 1668-74.
-
Smedman A, Vessby B: Conjugated linoleic acid supplementation in humans - Metabolic effects. Journal of Nutrition. 2001, 36: 773-81.
-
Tsuboyama-Kasaoka N, Takahashi M, Tanemura Grand, Kim HJ, Tange T, Okuyama H, Kasai Grand, Ikemoto SS, Ezaki O: Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000, 49: 1534-42. ten.2337/diabetes.49.9.1534.
-
Clement 50, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief B, Staels B, Besnard P: Dietary trans-ten, cis-12 conjugated linoleic acrid induces hyperinsulemia and fatty liver in the mouse. Periodical of Lipid Research. 2002, 43: 1400-9. 10.1194/jlr.M20008-JLR200.
-
Roche HM, Noone East, Sewter C, McBennett S, Savage D, Gibney MJ, O'Rahilly S, Vidal-Plug AJ: Isomer-dependent metabolic effects of conjugated linoleic acrid: insights from molecular markers sterol regulatory chemical element-bounden protein 1c and LXR blastoff. Diabetes. 2002, 51: 2037-44. 10.2337/diabetes.51.seven.2037.
-
Riserus U, Arner P, Brismar K, Vessby B: Treatment with dietary trans 10 cis 12 conjugated linoleic acid causes isomer specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002, 25: 1516-21. 10.2337/diacare.25.9.1516.
-
Delany JP, Blohm F, Truett AA, Scimeca JA, West DB: Conjugated linoleic acrid rapidly reduces trunk fat content in mice without affecting energy intake. American Journal of Physiology. 1999, 276 (4 pt 2): R1172-R1179.
-
Kelley DS, Simon VA, Taylor PC, Rudolph IL, Benito P: Dietary supplementation with conjugated linoleic acid increased its concentration in human peripheral blood mononuclear cells, but did not alter their function. Lipids. 2001, 36: 669-74. 10.1007/s11745-001-0771-z.
-
Whigham LD, Cook ME, Atkinson RL: Conjugated linoleic acrid: Implications for homo health. Pharmacological Research. 2000, 42 (6): 503-10. 10.1006/phrs.2000.0735.
-
Schmid A, Collomb Thou, Sieber R, Bee G: Conjugated linoleic acid in meat and meat products. A review Meat Scientific discipline. 2006, 73: 29-41. 10.1016/j.meatsci.2005.10.010.
-
Knekt P, Jarvinen R, Seppanen R, Pukkala E, Aromaa A: Intake of dairy products and the take a chance of breast cancer. British Journal of Cancer. 1996, 73: 687-91.
-
Ha YL, Grimm NK, Pariza MW: Newly recognized anticarcinogenic fatty acids: identification and quantification in natural and processed cheese. Journal of Agronomical and Food Chemistry. 1989, 37: 75-81. x.1021/jf00085a018.
-
Ritzenthaler KL, McGuire MK, Falen R, Shultz TD, Dasgupta Due north, McGuire MA: Estimation of conjugated linoleic acid intake by written dietary cess methodologies underestimates actual intake evaluated by food duplicate methodology. Journal of Nutrition. 2001, 131: 1548-54.
-
Parodi PW: Conjugated linoleic acid: an anticarcinogenic fatty acid present in milk fatty (review). Australian Journal of Dairy Technology. 1994, 49 (2): 93-seven.
-
Dunne PG, Monahan FJ, O'Mara FP, Moloney AP: Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Science. 2009, 81 (1): 28-45. 10.1016/j.meatsci.2008.06.013.
-
Chauveau-Duriot B, Thomas D, Portelli J, Doreau Grand: Carotenoids content in forages: variation during conservation. Renc Rech Ruminants. 2005, 12: 117-
-
Scott LW, Dunn JK, Pownell HJ, Brauchi DJ, McMann MC, Herd JA, Harris KB, Savell JW, Cantankerous HR, Gotto AM: Effects of beef and craven consumption on plasma lipid levels in hypercholesterolemic men. Archives of Internal Medicine. 1994, 154 (11): 1261-7. 10.1001/archinte.154.eleven.1261.
-
Hunninghake DB, Maki KC, Kwiterovick PO, Davidson MH, Dicklin MR, Kafonek SD: Incorporation of lean blood-red meat National Cholesterol Teaching Program Stride I diet: a long-term, randomized clinical trial in free-living persons with hypercholesterolemic. Journal of American Colleges of Nutrition. 2000, 19 (3): 351-60.
-
National Institute of Health Clinical Nutrition Center: Facts about dietary supplements: Vitamin A and Carotenoids. 2002, Ref Blazon: Pamphlet
-
Descalzo AM, Insani EM, Biolatto A, Sancho AM, Garcia PT, Pensel NA, Josifovich JA: Influence of pasture or grain-based diets supplemented with vitamin Eastward on antioxidant/oxidative balance of Argentine beef. Journal of Meat Science. 2005, 70: 35-44. 10.1016/j.meatsci.2004.11.018.
-
Simonne AH, Light-green NR, Bransby DI: Consumer acceptability and beta-carotene content of beef as related to cattle finishing diets. Periodical of Food Scientific discipline. 1996, 61: 1254-6. 10.1111/j.1365-2621.1996.tb10973.x.
-
Duckett SK, Pratt SL, Pavan E: Corn oil or corn grain supplementation to stters grazing endophyte-free tall fescue. II. Effects on subcutaneous fat acrid content and lipogenic gene expression. Journal of Animal Science. 2009, 87: 1120-8. x.2527/jas.2008-1420.
-
Yang A, Brewster MJ, Lanari MC, Tume RK: Issue of vitamin Eastward supplementation on alpha-tocopherol and beta-carotene concentrations in tissues from pasture and grain-fed cattle. Meat Science. 2002, 60 (1): 35-40. x.1016/S0309-1740(01)00102-4.
-
Pryor WA: Vitamin Due east and Carotenoid Abstracts- 1994 Studies of Lipid-Soluble Antioxidants. Vitamin East Enquiry and Information Services. 1996
-
Arnold RN, Scheller Northward, Arp KK, Williams SC, Beuge DR, Schaefer DM: Effect of long or short-term feeding of alfa-tocopherol acetate to Holstein and crossbred beef steers on performance, carcass characteristics, and beef color stability. Journal Fauna Science. 1992, 70: 3055-65.
-
Descalzo AM, Sancho AM: A review of natural antioxidants and their furnishings on oxidative status, smell and quality of fresh beef in Argentina. Meat Science. 2008, 79: 423-36. 10.1016/j.meatsci.2007.12.006.
-
Insani EM, Eyherabide A, Grigioni G, Sancho AM, Pensel NA, Descalzo AM: Oxidative stability and its human relationship with natural antioxidants during refrigerated retail brandish of beef produced in Argentina. Meat Science. 2008, 79: 444-52. ten.1016/j.meatsci.2007.10.017.
-
Lonn EM, Yusuf Due south: Is there a function for antioxidant vitamins in the prevention of cardiovascular diseases? An update on epidemiological and clinical trials information. Cancer Periodical of Cardiology. 1997, thirteen: 957-65.
-
Jialal I, Fuller CJ: Effect of vitamin E, vitamin C and beta-carotene on LDL oxidation and atherosclerosis. Canadian Journal of Cardiology. 1995, 11 (supplemental G): 97G-103G.
-
Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC: Vitamin E consumption and the chance of coronary disease in women. New England Journal of Medicine. 1993, 328 (1444): 1449-
-
Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A: Antioxidant vitamin intake and coronary mortality in a longitudinal population study. American Periodical of Epidemiology. 1994, 139: 1180-9.
-
Weitberg AB, Corvese D: Effects of vitamin E and beta-carotene on Deoxyribonucleic acid strand breakage induced by tobacco-specific nitrosamines and stimulated homo phagocytes. Periodical of Experimental Cancer Research. 1997, 16: 11-4.
-
Leske MC, Chylack LT, He Q, Wu SY, Schoenfeld East, Friend J, Wolfe J: Antioxidant vitamins and nuclear opacities: The longitudinal study of cataract. Ophthalmology. 1998, 105: 831-half-dozen. 10.1016/S0161-6420(98)95021-7.
-
Teikari JM, Virtamo J, Rautalahi M, Palmgren J, Liestro Thou, Heinonen OP: Long-term supplementation with alpha-tocopherol and beta-carotene and historic period-related cataract. Acta Ophthalmologica Scandinavica. 1997, 75: 634-forty. 10.1111/j.1600-0420.1997.tb00620.x.
-
Dietary guidelines Informational Committee, Agricultural Research Service Us Section of Agriculture USDA: Study of the dietary guidelines informational committee on the dietary guidelines for Americans. Dietary guidelines Advisory Committee. 2000, Ref Blazon: Hearing
-
McClure EK, Belk KE, Scanga JA, Smith GC: Determination of appropriate Vitamin East supplementation levels and assistants times to ensure adequate muscle tissue blastoff-tocopherol concentration in cattle destined for the Nolan Ryan tender-aged beef programme. Animal Sciences Inquiry Report. 2002, The Section of Brute Sciences, Colorado Country University, Ref Type: Report
-
Yang A, Lanari MC, Brewster MJ, Tume RK: Lipid stability and meat colour of beef from pasture and grain-fed cattle with or without vitamin E supplement. Meat Science. 2002, 60: 41-l. x.1016/S0309-1740(01)00103-6.
-
Valencia E, Marin A, Hardy Grand: Glutathione - Nutritional and Pharmacological Viewpoints: Part II. Nutraceuticals. 2001, 17: 485-six.
-
Valencia Due east, Marin A, Hardy G: Glutathione - Nutritional and Pharmacologic Viewpoints: Office 4. Nutraceuticals. 2001, 17: 783-4.
-
Descalzo AM, Rossetti L, Grigioni G, Irurueta Yard, Sancho AM, Carrete J, Pensel NA: Antioxidant status and odor profile in fresh beef from pasture or grain-fed cattle. Meat Science. 2007, 75: 299-307. ten.1016/j.meatsci.2006.07.015.
-
Gatellier P, Mercier Y, Renerre M: Effect of nutrition finishing mode (pasture or mixed diet) on antioxidant condition of Charolais bovine meat. Meat Science. 2004, 67: 385-94. 10.1016/j.meatsci.2003.11.009.
-
French P, O'Riordan EG, Monahan FJ, Caffery PJ, Moloney AP: Fatty acid composition of intra-muscular tricylglycerols of steers fed fall grass and concentrates. Livestock Production Science. 2003, 81: 307-17. 10.1016/S0301-6226(02)00253-1.
-
Elmore JS, Warren HE, Mottram DS, Scollan ND, Enser Chiliad, Richardson RI: A comparison of the aroma volatiles and fat acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed deits based on silage or concentrates. Meat Science. 2006, 68: 27-33. 10.1016/j.meatsci.2004.01.010.
-
Lorenz Due south, Buettner A, Ender K, Nuernberg M, Papstein HJ, Schieberle P: Influence of keeping system on the fatty acid limerick in the longissimus muscle of bulls and odorants formed after pressure-cooking. European Food Enquiry and Technology. 2002, 214: 112-8. 10.1007/s00217-001-0427-4.
-
Calkins CR, Hodgen JM: A fresh look at meat flavor. Meat Science. 2007, 77: 63-80. x.1016/j.meatsci.2007.04.016.
-
Sanudo C, Enser ME, Campo MM, Nute GR, Maria One thousand, Sierra I, Wood JD: Fat acrid limerick and sensory characteristics of lamb carcasses from Britain and Espana. Meat Science. 2000, 54: 339-46. 10.1016/S0309-1740(99)00108-four.
-
Killinger KM, Calkins CR, Umberger WJ, Feuz DM, Eskridge KM: A comparing of consumer sensory acceptance and value of domestic beef steaks and steaks form a branded, Argentine beef program. Periodical Animal Scientific discipline. 2004, 82: 3302-7.
Acknowledgements
The authors would like to acknowledge Grace Berryhill for her assistance with the figures, tables and editorial contributions to this manuscript.
Writer information
Authors and Affiliations
Corresponding author
Boosted data
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CAD was responsible for the literature review, completed most of the primary writing, created the manuscript and worked through the submission process; AA conducted the literature search, organized the articles according to category, completed some of the chief writing and served as editor; SPD conducted a portion of the literature review and served every bit editor for the manuscript; GAN conducted a portion of the literature review and served as editor for the manuscript; SL conducted a portion o the literature review and served as editor for the manuscript. All authors read and approved the concluding manuscript.
Authors' original submitted files for images
Rights and permissions
This commodity is published under license to BioMed Fundamental Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted utilize, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
About this commodity
Cite this article
Daley, C.A., Abbott, A., Doyle, P.South. et al. A review of fat acrid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr J 9, x (2010). https://doi.org/10.1186/1475-2891-9-10
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1475-2891-9-10
Keywords
- Conjugated Linoleic Acid
- Conjugated Linoleic Acrid Isomer
- Antioxidant Content
- Total Conjugated Linoleic Acid
- Conjugated Linoleic Acrid C9t11
mcfarlaneexpriver.blogspot.com
Source: https://nutritionj.biomedcentral.com/articles/10.1186/1475-2891-9-10